Abstract
Background:
Autoimmune neutropenia (AIN) is the most common chronic neutropenia of childhood. Despite the expectation of a benign clinical course, many such patients undergo extensive evaluation to rule out congenital neutropenia or malignant disease, are followed within a subspecialty hematology clinic until resolution, and are given strict infectious precautions including emergency center (EC) assessment during febrile episodes. Data on healthcare utilization and rate of serious infections in young patients with AIN is limited.
Objective:
To evaluate the utilization of routine subspecialty and emergency healthcare and incidence of serious bacterial infections in young children with AIN at a large tertiary care children's hospital.
Methods:
All patients with a diagnosis code of leukopenia, neutropenia, or AIN followed within the outpatient hematology clinic of a large tertiary hospital from 2014 to 2016 were identified. Manual review of the electronic medical record was performed to assess patient eligibility and perform data collection. Patients age ≤5 years, with absolute neutrophil count (ANC) ≤500/µL persisting for ≥3 months, and a clinical diagnosis of AIN as determined by a hematology provider were included. Patients were excluded if they were lost to follow up prior to neutropenia resolution, had AIN secondary to underlying systemic autoimmune or immunologic disorder, neutropenia persisting beyond age 5 years, or if the etiology of neutropenia was unclear. Data on clinical assessment, laboratory parameters, immune and infectious evaluations, and total number of outpatient and EC assessments for fever were collected. Results of anti-neutrophil antibody testing were reviewed but no patients were included or excluded based on those results. EC visits for evaluation of fever in 3 representative patients were reviewed for charge capture data. All charges during the reviewed time period remained constant.
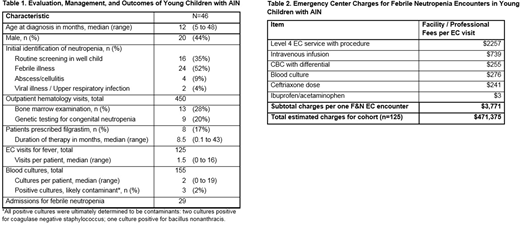
Results:
From 2014 to 2016, 46 patients (20 male [44%], median age at diagnosis 12 months) diagnosed with AIN met eligibility criteria and were followed in the outpatient hematology clinic for a total of 450 outpatient visits (Table 1). Median duration from initial identification of neutropenia to discharge from hematology clinic was 19 months (range 6 to 85 months). Thirteen patients (28%) had bone marrow evaluation to rule out infiltrative disease or marrow failure etiologies. Nine patients (20%) had genetic testing, the majority of which (n=6) were targeted sequencing for congenital neutropenia. Thirty-six patients (78%) had one or more EC encounters for evaluation of fever, and the cohort had a combined 125 such encounters. Of 155 blood cultures drawn, 3 returned positive. Positive cultures resulted in repeat blood cultures being drawn and hospital admission for antibiotics and clinical monitoring. However, all were determined to be contaminants that did not require treatment. Total charges associated with EC visits for the cohort are estimated at $471,375 based on a cost of $3771 per visit (Table 2). Those patients with ANC <500/µL at the time of presentation with isolated fever were admitted for observation per hospital policy, resulting in 29 inpatient hospitalizations after initial hematology visit.
Conclusion:
Over a 3-year period, no serious bloodstream or life-threatening infections were identified in a cohort of 46 young children with AIN presenting to the EC for assessment of fever at a single, large tertiary care children's hospital. A conservative estimate of EC charges for this cohort totaled just under half a million dollars for all visits in the study period. Costs would be expected to vary by center, and additional intangible costs include parental and patient anxiety, travel costs, and missed school or work. Further prospective research is needed to determine the potential for shifting toward a less intensive, more cost-effective management paradigm among young children with AIN without compromising patient safety.
Despotovic:Novartis: Research Funding; AmGen: Research Funding; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal